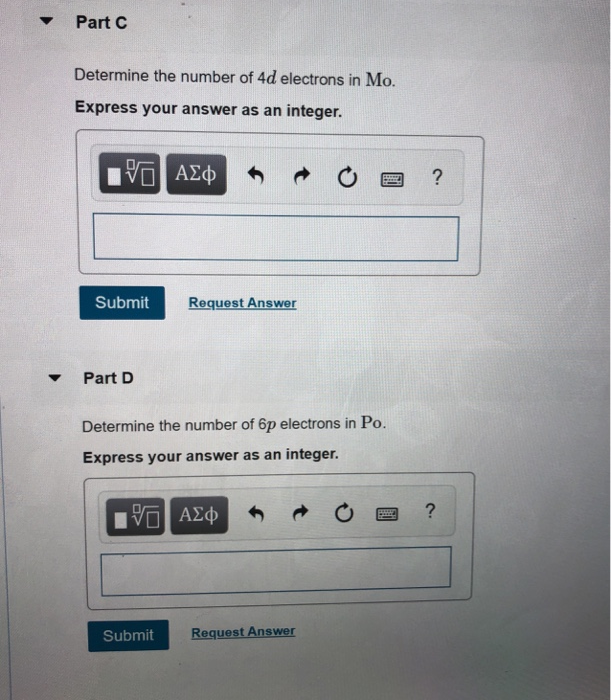
The Number of 4d Electrons in Mo.
The electrons in the 4d55s1 constitute its valence electrons. For Mo 3 ionization of transition metals always starts from the valence level the highest principle Quantum Number 5 then moves into the 4d level.
Solved Exercise 8 48 Enhanced With Feedback Part A Chegg Com
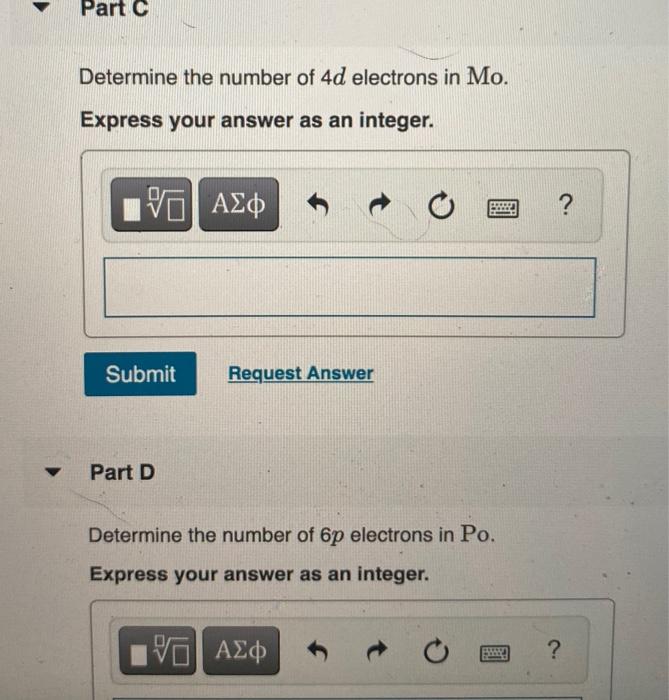
Express your answer as an integer______The number of 6p electrons in Po.

. How many 4d electrons does a ground-state Mo atom have. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 4 But in reality one electron moves from the 5s orbital to the 4d orbital. Asked Aug 24 2019 in Chemistry by Ashley.
Mo3 Kr 4d3. Express your answer as an integer______The number of 3s electrons in Na Express your answer as an integer_____The number of 3d electrons in V. Number of Neutrons most commonstable nuclide.
For Mo it is in the 5th row of the periodic table Mo. Number of Electrons with no charge. The number of 3d electrons in Cr.
When electrons get promoted from the Highest occupied molecular orbital MO to the Lowest unoccupied MO they are electrons of higher energy. There are 5 total 4 d orbitals. 1s2 2s2p6 3s2p6d10 4s2p6d1 5s2.
The electronic configuration of ground state Mo is. Determine the number of 3d electrons in Cr 2. The electrons in the 4d55s1 constitute its valence electrons.
D More questions like this. Normally when atoms get ionized they lose the electron that has the highest energy level. Express your answer as an integer_____.
Electrons per Energy Level. Chromium Cr has an electronic configuration of Ar 3d 5 4s 1. The symbol for molybdenum is Mo.
Solved Locate In The Periodic Table The Element That Has The Last 4d Electron Ar B Cd C K D Mo Which Of The Following Electrons Is Degenerate Having The Same Energy With An. Electron configurations pour it until it is full orbitals and also valence electrons of 4d elements. Chemistry questions and answers.
Find step-by-step Chemistry solutions and your answer to the following textbook question. The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. Looking upon this we see that 4d orbital holds 5 electrons.
What is the Maximum number of electrons into 4d orbital. Name an element in the fifth period row of the periodic table with five valence electrons. The number of 6p electrons in Pb.
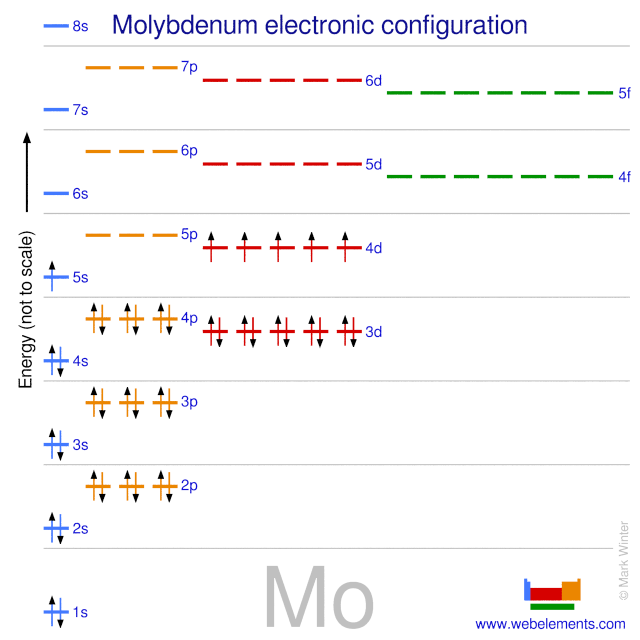
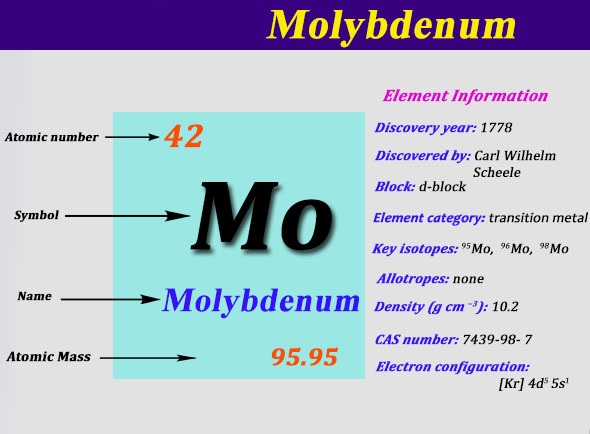
4d 5 5s 1 Electron Dot Model. The number of 4d electrons in Y. The atomic number of molybdenum is 42 and its electron configuration is 1s22s22p63s23p63d104s24p64d55s1 or 2 8 18 13 1 electrons per shell.
Solved The Number Of 4d Electrons In Y Express Your Answer Chegg Com. Answered Aug 24 2019 by meghanhollier. If you are removing electrons the first to come out is in the 5s electrons since transition metals lose s electrons before d electrons.
Chemistry questions and answers. 1s2 2s2p6 3s2p6d10. 43 protons and 43 electrons as its atomic number is 43For the most stable isotope Tc-98 there are 55 neutrons 98 - 43 55 How many protons and neutrons and electrons are in technetium.
This rule is known as. The number of 4d electrons in Y. Chemical Properties of Molybdenum.
Department of Energys Office of Scientific and Technical Information. Chemistry questions and answers. The number of 3d electrons in Cr.
4d 4 5s 1. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1 4d 5. Molybdenum atoms have 42 electrons and the shell structure is 2818131.
The number of 4d electrons in Y. It shows the order of electron entry for the building up process. Determine the number of 6p electrons in Po 4.
Looking upon this we see that 3d orbital holds 5 electrons. Express your answer as an integer. 1s2 2s2p6 3s2p6d10 4s2p6d2 5s2.
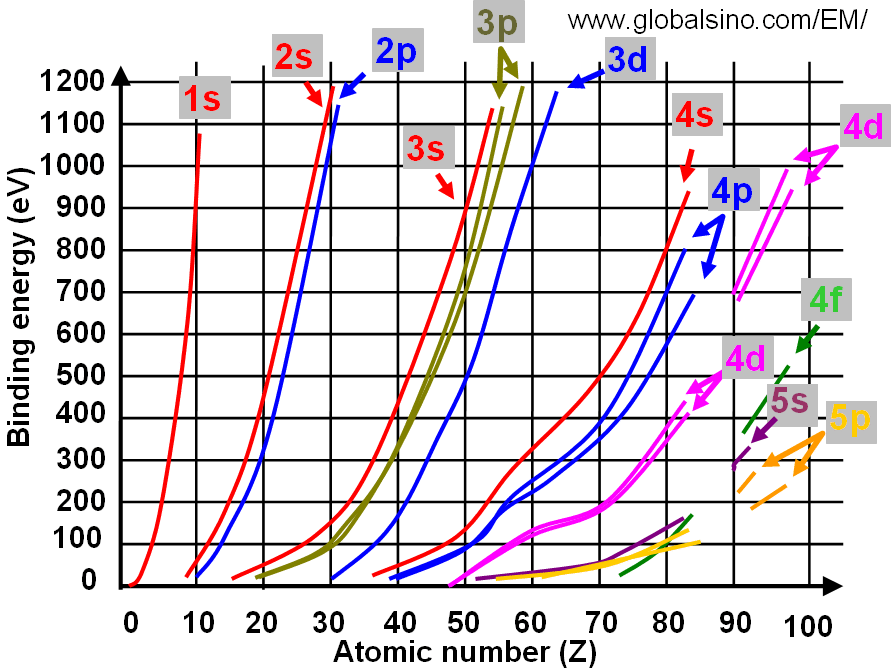
For Mo it is in the 5th row of the periodic table Mo. 11 rows Electron configurations filling orbitals and valence electrons of 4d elements. However even though the 5s orbital is lower in energy than the 4d orbital the electrons in the 4d orbitals shield the electron.
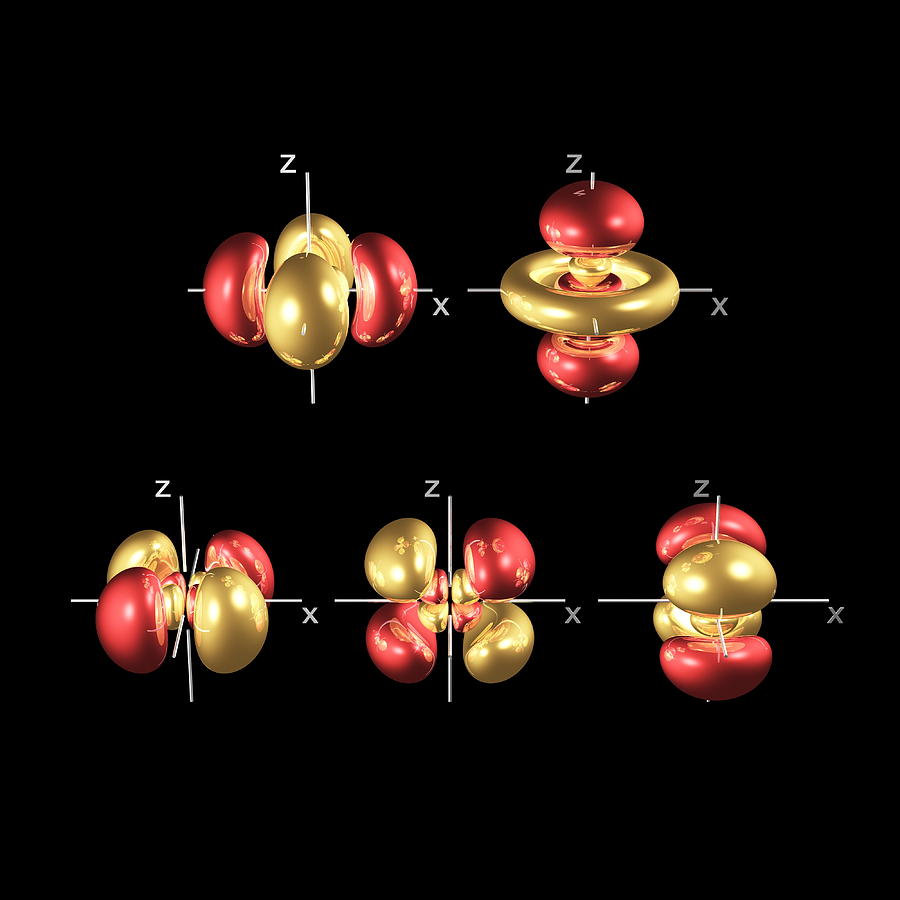
Or in condensed form Kr4d55s1. If you are filling in the electrons it will be in the 4d orbital. 4dy 4dx 4dz 4dz2 4dx2-y2Each of these can fit 2 electrons.
5s1 and the term symbol is 7S3. The number of 3s electrons in Mg. Chemistry questions and answers.
The number of 3s electrons in Mg. Use the periodic table to determine each quantity. Determine the number of 4d electrons in Mo 3.
Atom Number Symbol name Electron configuration Filling orbit Valence electrons. The ground state electron configuration of ground state gaseous neutral molybdenum is Kr. Electronic configuration of the Molybdenum atom in ascending order of orbital energies.
Lead Pb has an electronic configuration of Xe 4f 14 5d 10 6s 2 6p 6. Molybdenum Mo has an electronic configuration of Kr 4d 5 5s 1.
Webelements Periodic Table Molybdenum Properties Of Free Atoms

Light And The Modern Atom Teaching Chemistry Chemistry Education Physics And Mathematics

Molybdenum Deficiencies Health Benefits Food Sources Electron Configuration Chromium Energy Level
4d Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book
Solved The Number Of 4d Electrons In Y Express Your Answer Chegg Com
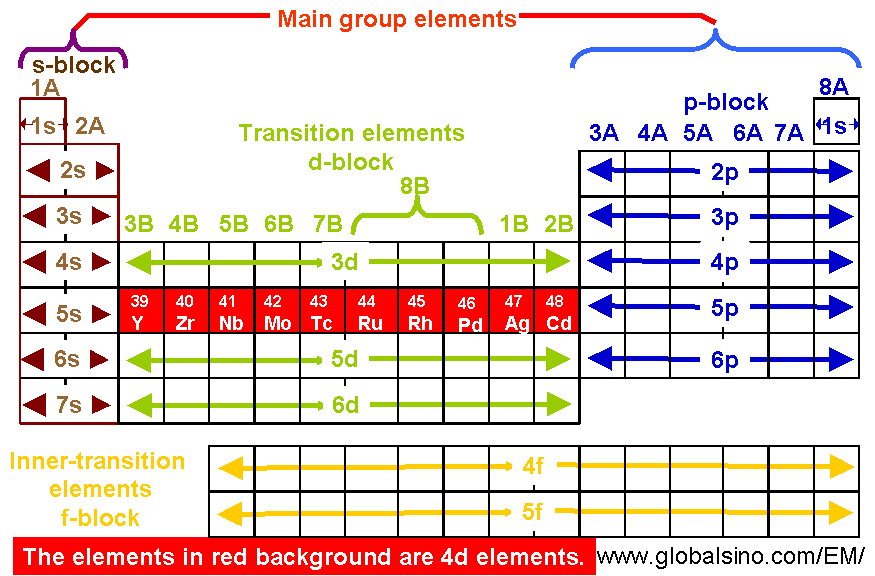
4d Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book
Solved Determine The Number Of 3s Electrons In Mg Express Chegg Com

White S Periodic Table 1934 Periodic Table Internet Database Physical Science
Molybdenum Electron Configuration Mo With Orbital Diagram

28 How Many 4d Electrons Are In An Atom Of Each Of The Following Elements A Homeworklib

Solved Locate In The Periodic Table The Element That Has The Last 4d Electron Ar B Cd C K D Mo Which Of The Following Electrons Is Degenerate Having The Same Energy With An

Depending On The Ionization Energy Of Oxygen Meaning How Many Electrons It Might Have 1 314 Kj Mol 3 388 Kj Mol Ionization Energy Periodic Table Chemistry

Periodic Table Of The Elements Janet Form Periodic Table Of The Elements Periodic Table Physics
4d Electron Orbitals Photograph By Dr Mark J Winter
Solved U Review Part A Determine The Number Of 3s Electrons Chegg Com

Which Of The Following Represents The Correct Set Of The Four Quantum Numbers Of 4d Electrons

28 How Many 4d Electrons Are In An Atom Of Each Of The Following Elements A Homeworklib

In The Predicted Electron Configuration Of Molybednum Mo How Many Electrons Are In The 4d Subshell Homeworklib

Comments
Post a Comment